


Hsp70 Chaperone binden überwiegend ungefaltete Polypeptide, interagieren jedoch im allgemeinen nicht mit deren nativ gefalteten Formen. Diese Reaktionszyklen werden durch J-Proteine sowie Nukleotidaustauschfaktoren reguliert. Translation of abstract (German)Ĭhaperone der Hsp70-Familie sind an einer Vielzahl zellulärer Proteinfaltungsvorgänge beteiligt, indem sie über ATP verbrauchende Reaktionszyklen Substrate binden und freisetzen. I have been able to map the dimer interface in RepE and to identify the DnaK binding site which, interestingly, is not close to the dimer interface. I have characterized the molecular mechanism of this regulation by investigating the conformation of dimeric RepE wild type and the constitutively monomeric variant RepE54 by amide hydrogen exchange experiments. Monomerization of RepE is regulated by DnaK. RepE, on the other hand, performs different functions depending on its oligomeric state: as a dimer it represses its own synthesis while as a monomer it promotes replication initiation. DnaK destabilizes a region in the N-terminal domain, the primary target for the FtsH protease, which degrades s32 in vivo. DnaJ binding to s32 destabilizes a region in close spatial vicinity to the DnaK binding site, thereby explaining the catalytic action of DnaJ in loading s32 onto DnaK and the synergistic stimulation of DnaK’s ATPase activity by the simultaneous interaction of DnaJ and s32. I have been able to show that both chaperones influence the conformation of s32 by destabilizing specific regions distant to their binding sites. Using amide hydrogen exchange experiments combined with mass spectrometry, and deletion and point-mutation constructs, I have identified the DnaK and DnaJ binding sites in s32. coli Hsp70 homologue DnaK and its co-chaperone DnaJ with two protein substrates whose activity is regulated by DnaK and DnaJ: the heat-shock transcription factor s32 and the replication initiator protein RepE. I have analyzed the interaction of the E. The aim of this Thesis was to contribute to a deeper understanding of the Hsp70 interaction with natively folded substrates, studying their conformation and probing possible conformational changes due to the action of Hsp70. It is also unknown whether Hsp70 proteins keep their substrates in an unfolded conformation in solution or play a more active role by inducing conformational changes on them.
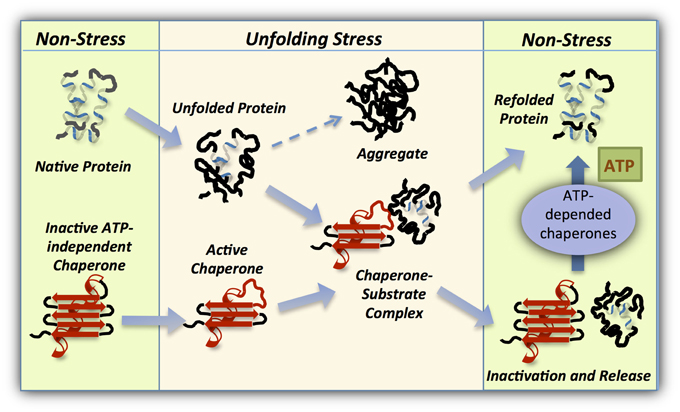
Even though the binding to peptide substrates has been extensively studied, it is still unclear how the binding to natively folded substrates occurs. However, Hsp70 also recognize certain folded proteins as substrates, like natively folded regulatory proteins, and modulates their activities. Hsp70 chaperones bind to almost all unfolded proteins but generally do not interact with their native counterparts. Hsp70 chaperones assist a large variety of protein folding processes in the cell by ATP-controlled cycles of substrate binding and release that are regulated by J-proteins and nucleotide exchange factors. Citation of documents: Please do not cite the URL that is displayed in your browser location input, instead use the DOI, URN or the persistent URL below, as we can guarantee their long-time accessibility.


 0 kommentar(er)
0 kommentar(er)
